Opportunities for Improved CAR T Cell Therapy
Published on 31 Aug, 2023
Research and technological advancements are targeting the limitations of chimeric antigen receptor T (CAR T) cell therapy to improve its efficiency, minimize side effects, and reduce manufacturing costs. Most of these technologies are under development and will take time to be implemented. Through these advancements, the future holds promising prospects to overcome the challenges associated with CAR T cell therapy.
Despite the successful application of CAR-T immunotherapy in patients for certain disorders or hematological malignancies, various obstacles necessitate resolution, including elevated relapse rates and undesirable side effects such as exhaustion, antigen escape, and on-target/off-tumor toxicity. Overcoming limitations of CAR T cell therapy, improving its efficacy, and reducing manufacturing time and costs are critical to take care of patients suffering from rapidly progressive diseases such as blood cancer and solid lung tumors.
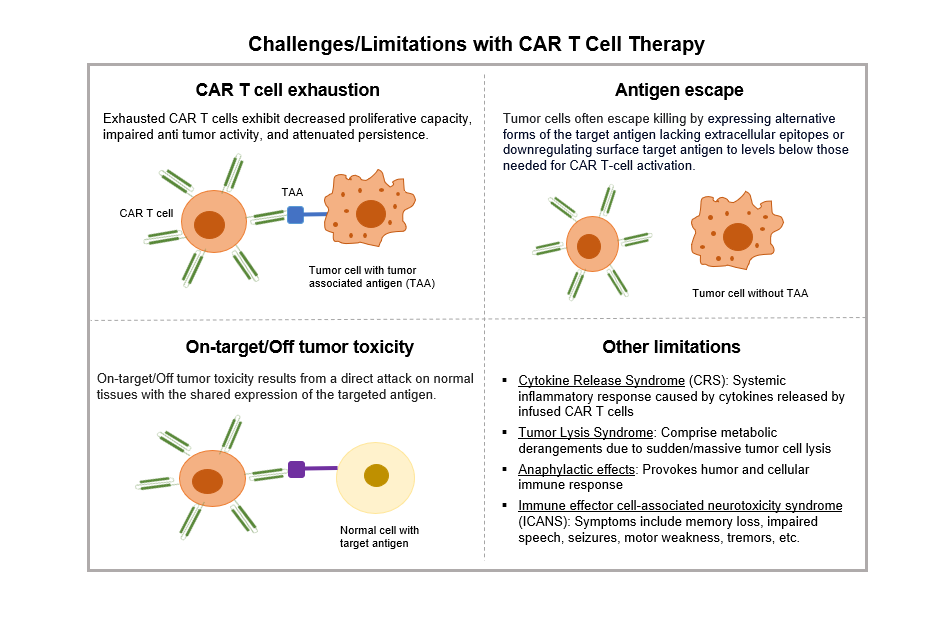
Several research groups are working on various elements of the technology and manufacturing process to overcome challenges that have been outlined.
Enhancing Efficacy of CAR T Cells
Combination therapies – CAR T cell therapy can be more effective with combination therapies. In this approach, CAR T cells are conjoined with an additional therapeutic entity, such as a checkpoint inhibitor, an oncolytic virus, or an RNA vaccine.
- Checkpoint inhibition: This process blocks the proteins that cancer cells use to escape immune surveillance. A notable illustration involves the PD-1 receptor, which acts as a checkpoint instructing T cells to refrain from attacking cells that exhibit the PD-L1 protein. However, certain tumor cells exhibit PD-L1, leading to antigen evasion. By blocking PD-1, CAR T cells can selectively target tumor cells that express PD-L1.
- Virus: Oncolytic viruses can rupture tumor cells, thereby releasing their antigenic contents and rendering them more recognizable to the patient's immune system, notably T cells. This modality of combination therapy demonstrated the potential to increase the efficacy of CAR T cell therapy within solid tumors, as demonstrated in a mouse model of melanoma.
- RNA vaccines: Preclinical trials in 2022 showed that the use of CAR T cells with RNA vaccines resulted in tumor regression in mice harboring human tumor grafts.
- mRNA: mRNA-based T cell modifications have emerged as a safe, expeditious, and cost-effective approach that circumvents challenges such as limited transgene capacity, protracted manufacturing processes, and the potential for uncontrollable off-tumor toxicities.
- Small molecules: Small-molecule compounds offer a potential solution to mitigate the various limitations encountered in CAR T cell therapy. These limitations encompass the expression of inhibitory receptors, diminished persistence, loss of target antigen recognition, occurrence of severe cytokine release syndrome (CRS), and neurotoxicity, among others.
- Hydrogel: Hydrogel-based regional delivery strategies are being developed to enhance the effectiveness of CAR T cell therapy against solid tumors. Due to their outstanding biocompatibility and biodegradability, injectable hydrogels permit the regional delivery of CAR T cells to enhance the tumor infiltration and persistence of the CAR T cells for the treatment of solid tumors.
- Nano-delivery systems: Research is being conducted to explore how conjugated nanoparticles to CAR T cells can overcome tumor microenvironment immunosuppression, enhancing T cell tumor infiltration. These nanocarriers can also be used to deliver therapeutic agents such as mRNA for enhancing efficacy of CAR T cells.
- Biomarkers – The identification of biomarkers for predicting the response or resistance to CAR T cell therapy is an ongoing area of opportunity. The development of robust biomarker signatures holds the potential to aid in the selection of suitable patients for treatment, anticipate treatment response, and inform therapeutic decision-making. Investigators from the Medical University of Vienna identified a highly potent biomarker, CD3+CD27-CD28--T cells, which may help identify lymphoma patients gain maximum benefit from CAR T cell therapy before infusion.
Creating Cost Efficiency in Manufacturing
Currently, manufacturing CAR T cell is an expensive proposition. One of the crucial stages in the manufacturing of CAR T cells involves their activation and expansion. To activate T cells, manufacturers devised artificial antigen presenting cells, such as microbeads coated with antibodies specifically targeting CD3+ T cells and CD28 co-stimulatory molecules. Initially, when CAR T cell therapy was in the nascent stage, microbeads were just coated with a single antibody for binding with CD3 to activate T cells. Subsequently, it was discovered that multi-epitope microbeads capable of displaying multiple antigens or epitopes yielded superior CAR T cell activation and expansion.
- Biodegradable microbeads: Biodegradable microbeads exhibit enhanced surface interactions with antigen-specific CD8+ T cells. This improved interaction leads to heightened T cell activation and expansion in vitro, which has been correlated with a notable increase in the efficiency of T cell stimulation signals. These advancements address the challenge posed by conventional microbeads in CAR T manufacturing, where incomplete removal may lead to potential side effects such as inflammation.
- Lentiviral delivery: Researchers at the University of Pennsylvania devised a method to circumvent the requirement for T cell activation during CAR T cell manufacturing. They accomplished this by directly delivering genes to non-activated T cells, which are freshly isolated from the bloodstream, using lentiviral vectors. There is a dual advantage of expediting the overall manufacturing process while preserving the potency of the T cells. The process reduces the manufacturing time from 1 to 2 weeks to a just 24 hours.
- Implantable devices: Preclinical investigations have demonstrated the potential of a novel implantable biotechnology, known as MASTER (Multifunctional Alginate Scaffolds for T cell Engineering and Release) to facilitate the production and in vivo release of CAR T cells. This innovative technology is capable of compressing the previously time-consuming multiweek procedure into a single-day process.
- Shape variable microbeads: The use of shape variable microbeads, along with their incorporation into biomaterial scaffolds, nanoparticles, or nanobeads, presents an additional avenue for achieving optimal interaction between an artificial antigen presenting cells and T cells. This interaction further facilitates efficient T cell activation and subsequent immune response.
Conclusion
The advancements in technologies and ongoing research focused on addressing the limitations of CAR T cell therapy have paved the way for promising solutions. There is considerable potential for enhancing the overall effectiveness, safety, and accessibility of CAR T cell therapy, benefiting patients and advancing cellular immunotherapy.

