CRISPR Therapeutics – Transforming Healthcare
Published on 12 Jun, 2023

CRISPR technology has been gaining prominence as a realistic treatment option for genetic diseases. Major recent licensing activity is seen from pharmaceutical companies that are also investing in startups in this domain. While technology could revolutionize healthcare, especially for genetic diseases, it has its shortcomings and research is ongoing to address them for wider adoption of CRISPR.
Clustered regularly interspaced short palindromic repeats (CRISPR) has emerged as a revolutionary treatment option for various diseases. The genome editing capabilities of CRISPR-associated nucleases was discovered less than a decade ago.
Over the past decade, CRISPR technology has undergone substantial growth. Since its discovery, CRISPR has been used to modify mammalian genomes, which has opened new avenues for the robust manipulation of the human genome for basic research and translational medicine. To date, 300 CRISPR-Cas nucleases have been characterized, and increasingly versatile, precise tools have been developed to make room for the numerous aspects of biological research and clinical application in treating disease. These tools have laid the groundwork for developing new therapeutic modalities for humans that can treat and/or eventually cure genetic diseases. Depending on the methodology adopted, CRISPR can be used to facilitate gene editing ex-vivo or in-vivo. CRISPRs are multicomponent systems that require packaging and delivery of a large protein, the gRNA, and expression-regulating elements.
- In an ex-vivo editing system, cells are extracted from the body, edited with engineered nucleases guided by CRISPR, and then reintroduced. This technique permits precise control.
- In in-vivo approaches, genome editing materials are delivered directly to diseased cells or organs.
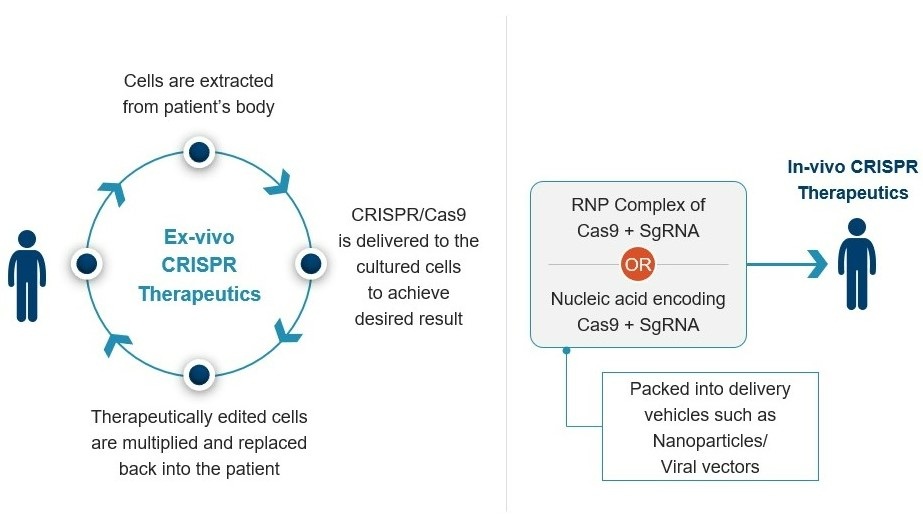
Figure 1: Depicting the delivery of CRISPR Therapy. (a) Ex-vivo therapeutics (b) In-vivo therapeutics.
Currently, several companies are conducting fundamental research on CRISPR/Cas variants to develop better gene editing solutions. Only a few pharmaceutical industry stakeholders are investigating the therapeutic applications of this versatile genetic manipulation tool. Companies such as Editas Medicine, CRISPR Therapeutics, and Intellia Therapeutics are using surrogate licensing to gain exclusive control of the associated intellectual property in the current market (Fig 2).

Figure 2: Intellectual property and licensing associated with CRISPR-based gene editing tool.
There is significant evidence validating the therapeutic applications of this technology, resulting in strategic partnerships between companies for therapy development and clinical research. A large amount of capital has been injected into innovator companies in this field over the past two years alone. The combined market capitalization of the three leading companies in this industry is more than USD 10 billion, and they have raised more than USD 2.8 billion through various funding rounds.
The discovery and development of the CRISPR/Cas9 system have provided gene therapy a second opportunity to overcome its stigma and prove to be an effective therapeutic strategy. Both autologous and allogenic CRISPR cell therapies work by editing mutations in the patient's own cells, and have the potential to circumvent the rejection problems associated with donor-matched transplant therapies. However, the production of autologous cell therapy takes several weeks, during which patients experience disease progression.
CRISPR genome editing is particularly promising for diseases treatable by autologous cell therapy. Extracting cells from a patient to edit the genome enables additional testing to prevent off-target genome modifications during genome editing.
Table 1: Ongoing/completed clinical studies for ex-vivo CRISPR-based therapies.
Future Prospects
Although ex-vivo gene editing produces highly effective cell therapies, it cannot be used to treat all genetic diseases. Certain dysfunctional cells and organs cannot be repaired by adding new/repaired cells to the body. Moreover, cell therapies frequently necessitate the elimination of patients' immune cells. Immunosuppression facilitates the proliferation of therapeutic cells; however, it poses a significant risk to the patient, rendering them susceptible to severe forms of common illnesses. Such rigorous preconditioning may prevent the administration of a second dose of certain cell therapies.
Thus, scientists are actively investigating in-vivo genome editing therapies. These therapies involve editing a large number of cells in situ, i.e., at their natural location in the body. They rely on technological advances that make it easier to deliver CRISPR to specific body parts. Such "in-vivo" genome editing may one day allow clinicians to treat and cure a plethora of untreatable or difficult-to-treat diseases.
CRISPR-Cas9 therapeutics, though in its infancy, is on the verge of ushering in the era of precision medicine.

